- Refer a Patient
- Referral Types
- Patient Information
- Overview of CFEH Clinics
- CFEH Instrument List
- Our clinical team
- Causes of Vision Loss
- Patient Forms





An extract from the CFEH Atlas
Retinitis Pigmentosa
Retinitis pigmentosa (RP) comprises a group of inherited retinal dystrophies characterised by the primary degeneration of rod and cone photoreceptors. It is the most common inherited retinal dystrophy with a worldwide prevalence of approximately 1 in 4000.
Over 90 genes have been implicated in RP with more added to the list each year. Autosomal recessive is the most common inheritance pattern account for 50-60% of RP patients, followed by autosomal dominant (30-40%) and X-linked (5-15%).
The initial symptom of RP is reduced night vision and difficulty with dark adaptation, followed by a progressive loss of the visual field in a concentric pattern and eventually loss of central vision. In the ‘classic’ presentation of RP, difficulty with dark adaptation begins in adolescence, and mid-periphery field loss becomes apparent in young adulthood. Typically, when the patient reaches middle age, central cone degeneration leads to a decline in visual acuity. Nevertheless, there is wide heterogeneity among RP patients differing in the age of onset, the severity of visual loss, and the rate of progression.
The hallmark features of RP include bone spicule pigmentation in the mid-periphery, attenuation of retinal vessels, and waxy pallor of the optic nerve head. However, these signs may be subtle or absent in the early stages.
Other associated clinical findings may include epiretinal membrane formation, posterior subcapsular cataract, macular hole formation and cystoid macular oedema. In early-onset RP, nystagmus and high refractive error are also common.
The earliest change on OCT shows disorganisation of outer retina layers, first in the interdigitation zone, followed by the ellipsoid zone, then the external limiting membrane, and finally the RPE. The inner nuclear layer and ganglion cell layers remain relatively well preserved in the early stages of the disease but degenerate later in the disease process.
The fundus autofluorescence pattern in RP typically shows hypo-fluorescence in the areas of the bone spicule pigmentation. There is a characteristic hyper-autofluorescent ring around the macula that separates the degenerated retina from the intact cells of the macula. The hyper-autofluorescence is thought to be caused by RPE cells under stress and is an indicator of imminent cell death. Over time, this ring constricts and moves closer to the fovea.
Visual field loss begins with isolated mid-peripheral scotomas, progressing to form a ring scotoma over time.
Colour vision may initially be normal; however, over time, blue-yellow colour vision defects typically evolve.
Full-field electroretinogram helps in the diagnosis, quantification of the severity of the disease, as well as monitoring the progression. It typically shows reduced scotopic responses followed by abnormal photopic responses. As the disease processes, the full-field ERG may become non-recordable despite a residual visual field.
Up to 30% of the patients present with a syndromic form of RP associated with extra-ocular abnormalities. The two most common of these are Usher Syndrome (associated neurosensory deafness) and Bardet Biedl syndrome (childhood obesity, developmental delays and renal abnormalities).
Case 1: "Typical RP
- Case 1: "Typical RP
- Case 2: Advanced RP
- Case 3: Unilateral RP
- Case 4: Sector RP
A 36-year-old Caucasian male with poor night vision and best-corrected visual acuity of 6/4.8- (20/15-) in each eye.
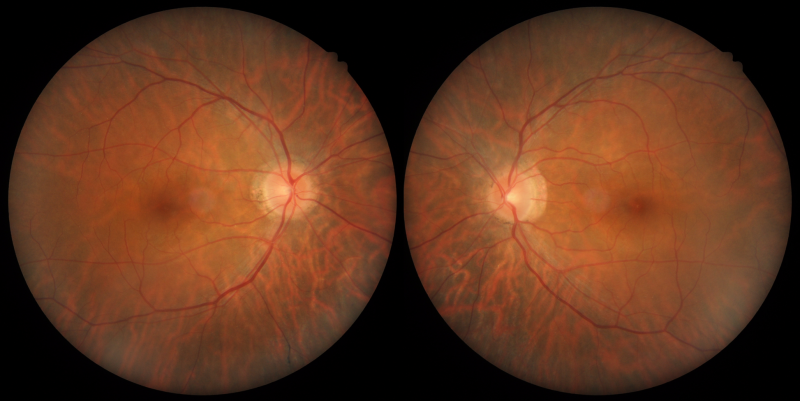
Colour fundus photographs do not reveal marked abnormality within the posterior pole.
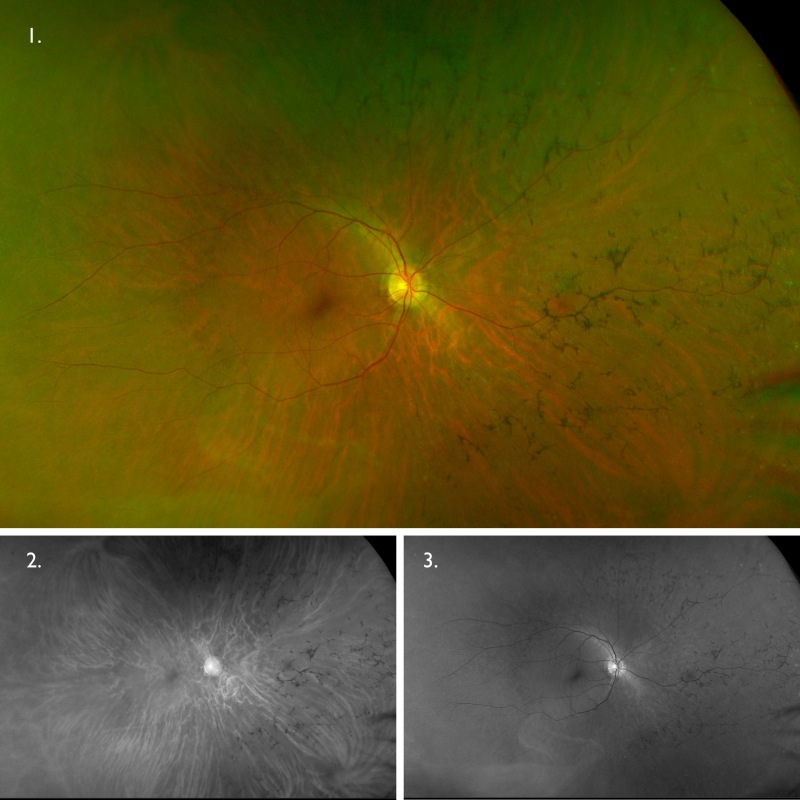
Optomap (1), red separation (2) and green separation (3) images – right eye: Widefield imaging shows bone spicule pigmentation in the nasal and superior mid-periphery.
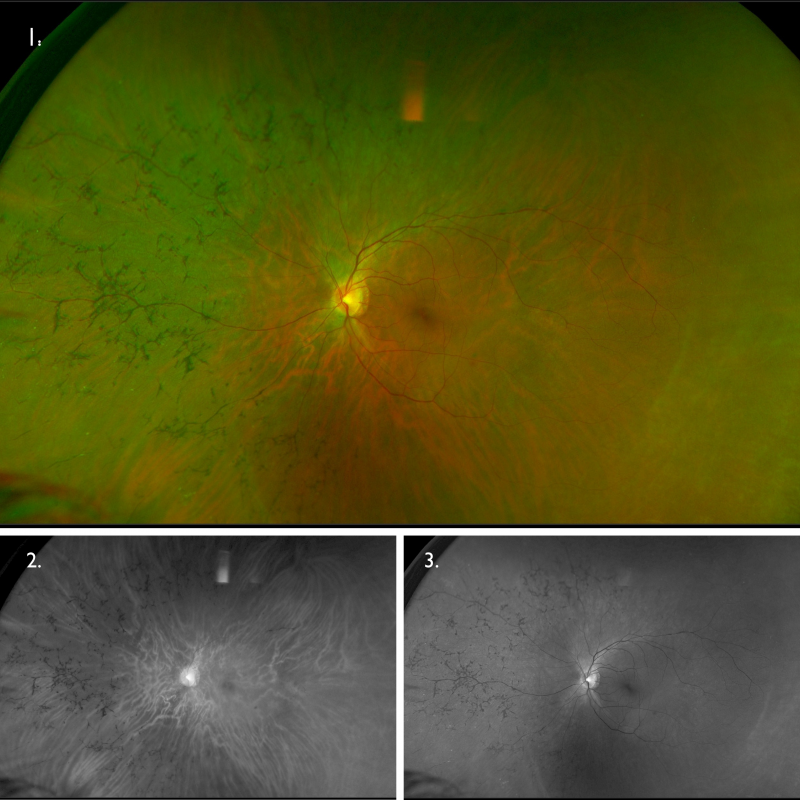
Optomap (1), red separation (2) and green separation (3) images – left eye: Widefield imaging shows bone spicule pigmentation in the nasal and superior mid-periphery.
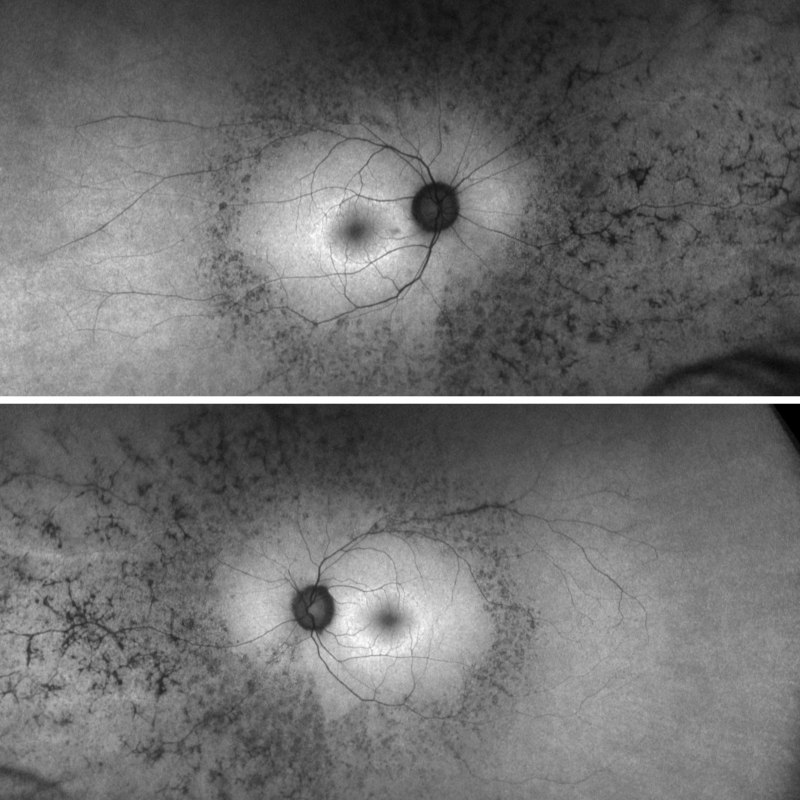
Fundus autofluorescence imaging: Autofluorescence imaging shows hypo-fluorescent changes corresponding to the areas of bone spicule pigmentation, with additional areas seen on FAF temporal to the macula. There is a hyper-autofluorescent ring centred in the macula that is characteristic of retinitis pigmentosa.
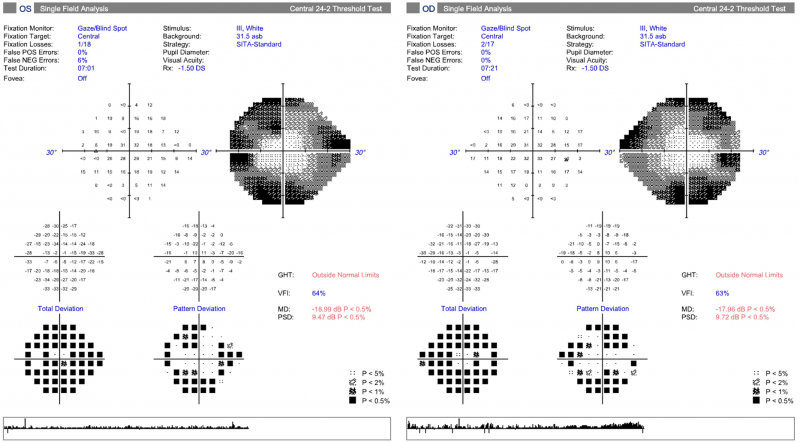
24-2 SITA Standard visual field: Central 24-2 field testing shows constriction of the fields bilaterally with the central and paracentral zones being relatively clear in each eye.
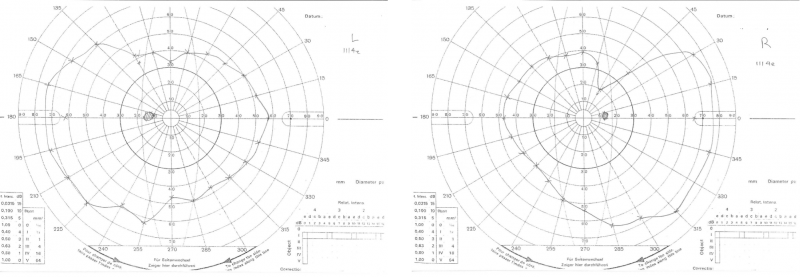
Goldmann kinetic perimetry: Kinetic perimetry using the III4e target shows constricted fields in both eyes, more notable in the right.
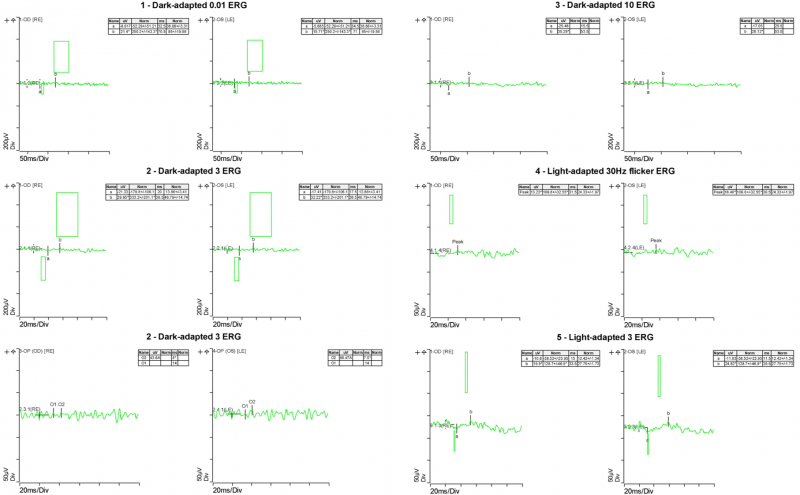
Electrophysiology results: Full-field ERG showed substantial reduction of photopic response amplitude and no detectable scotopic response. These results (taken in conjunction with the clinical findings) are consistent with a diagnosis of retinitis pigmentosa.
A 52-year-old Caucasian male with best-corrected visual acuity of 6/15 (20/50) in the right eye and 6/12- (20/40-) in the left. He reports difficulty with night vision and mobility.
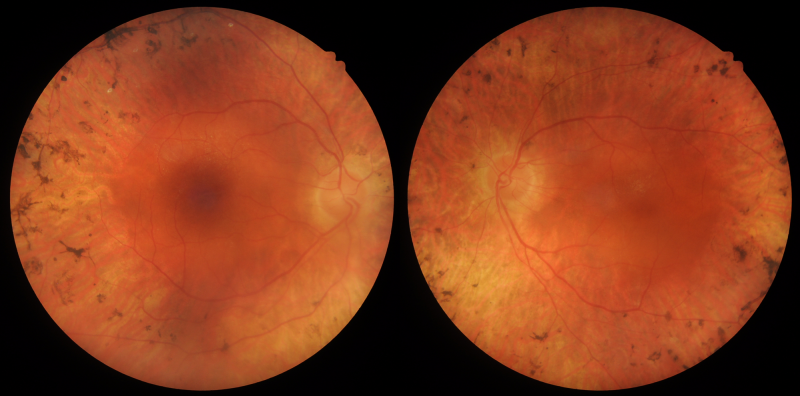
Optomap (1), red separation (2) and green separation (3) images – right eye: Widefield imaging shows widespread retinal atrophy and bone spicules.
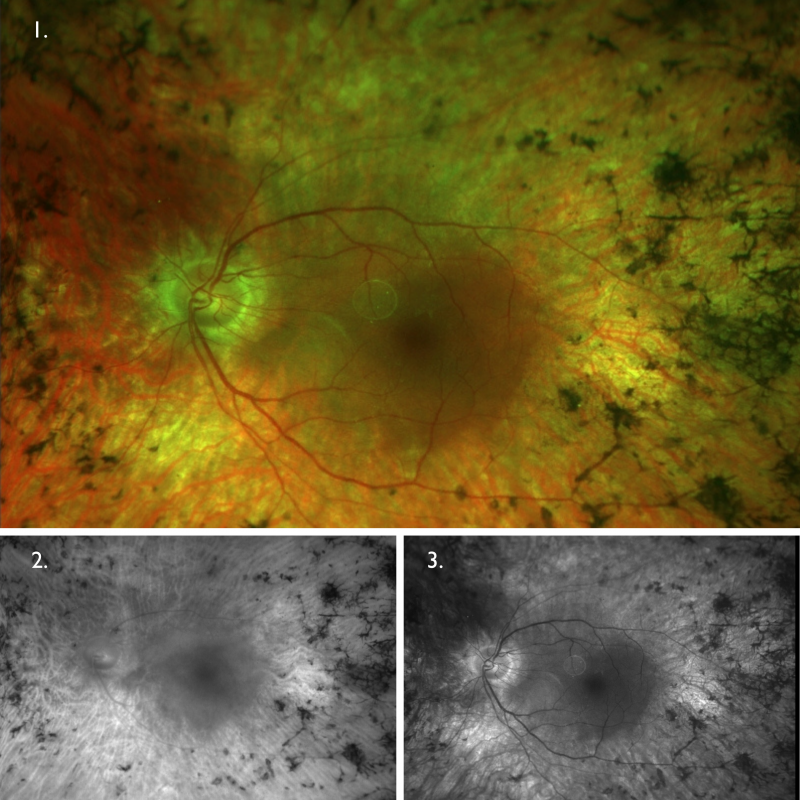
Optomap (1), red separation (2) and green separation (3) images – left eye: Widefield imaging shows widespread retinal atrophy and bone spicules.
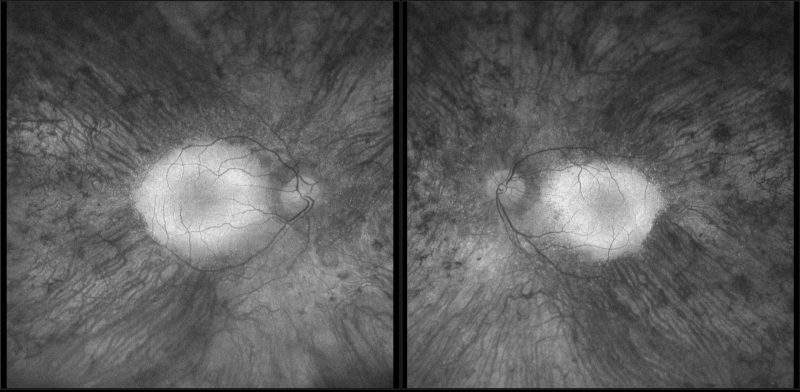
Fundus autofluorescence imaging: FAF shows a hyperfluorescent ring encompassing the macula and mottled hypo-autofluorescence in the periphery corresponding to widespread RPE atrophy.
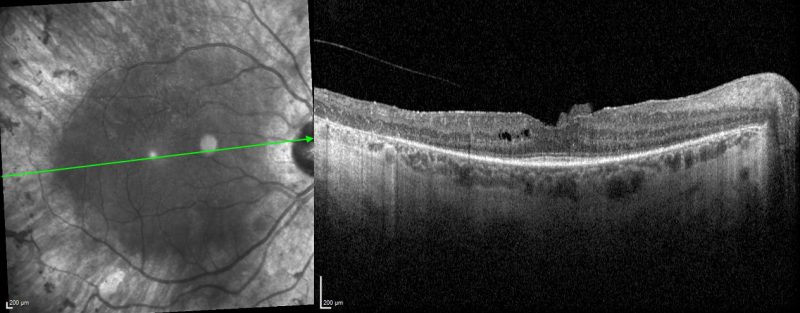
Spectralis OCT volume line scan (right macula): OCT shows significant disorganisation of the parafoveal outer retina including attenuation of outer nuclear layer, loss of interdigitation zone, ellipsoid zone and external limiting membrane, as well as RPE atrophy. Only a small subfoveal region is preserved. Cystoid macular oedema and epiretinal membrane can also be appreciated.
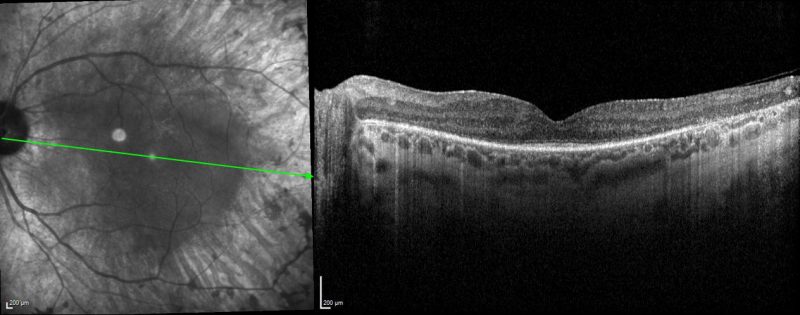
Spectralis OCT volume and line scans (left macula): OCT shows significant disorganisation of the parafoveal outer retina including attenuation of outer nuclear layer, loss of interdigitation zone, ellipsoid zone and external limiting membrane, as well as RPE atrophy. An epiretinal membrane is also present.
A 48-year-old Indian female with best-corrected visual acuity of 6/7.5 (20/25) in the right eye and 6/6+ (20/20+) in the left. The patient notes difficulty with her left peripheral vision.
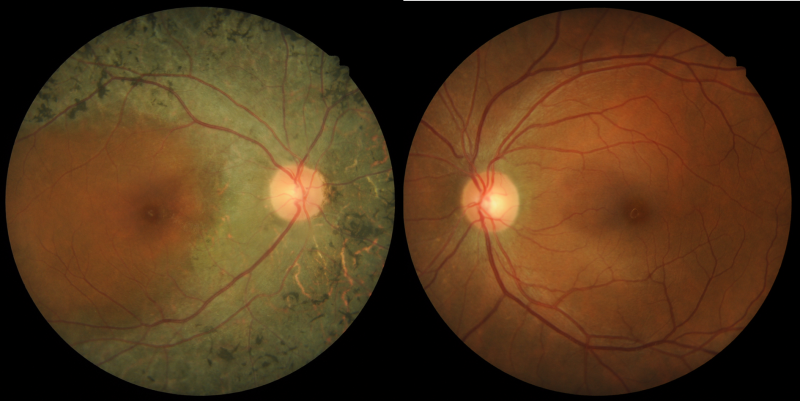
Colour fundus photographs: Fundus photographs show pigmentary abnormality along the vascular arcade and sparing the macula in the right eye. The left eye is unremarkable.
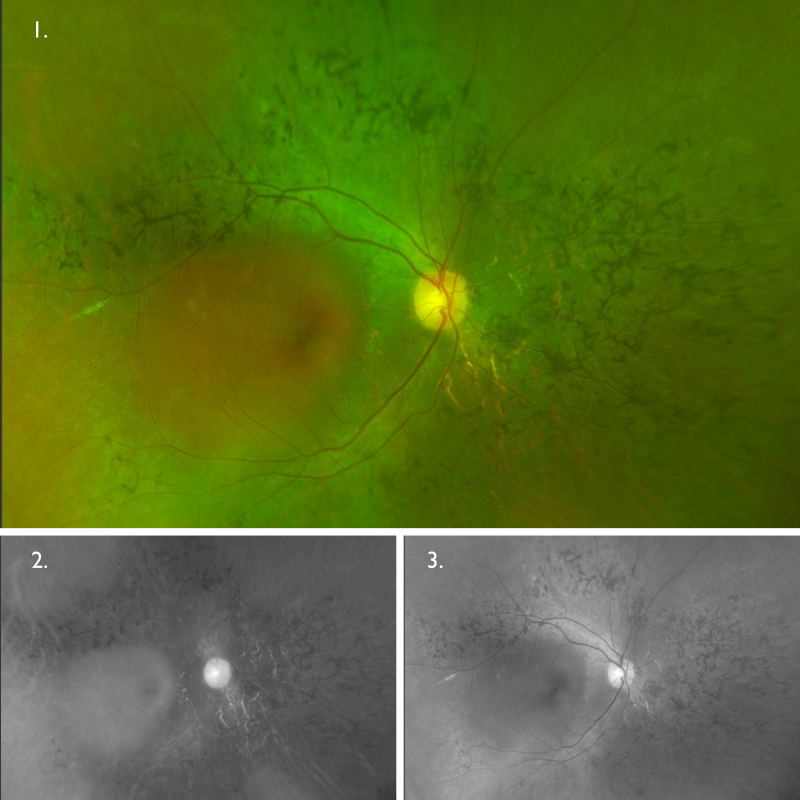
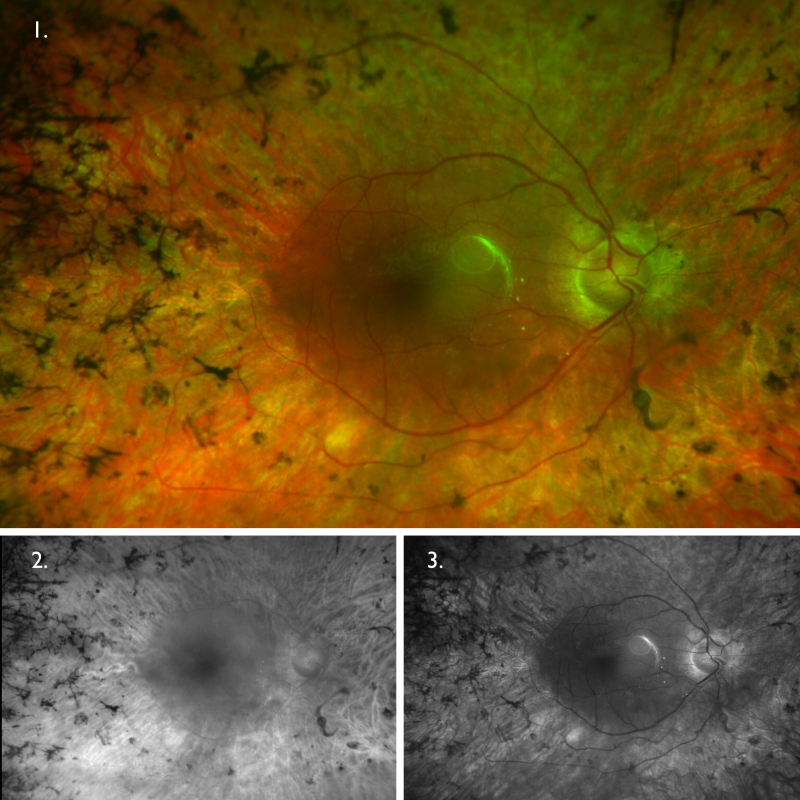
Optomap (1), red separation (2) and green separation (3) images – right eye: Widefield imaging of the right eye shows the mid-peripheral pigmentary changes, more notable nasally and superiorly.
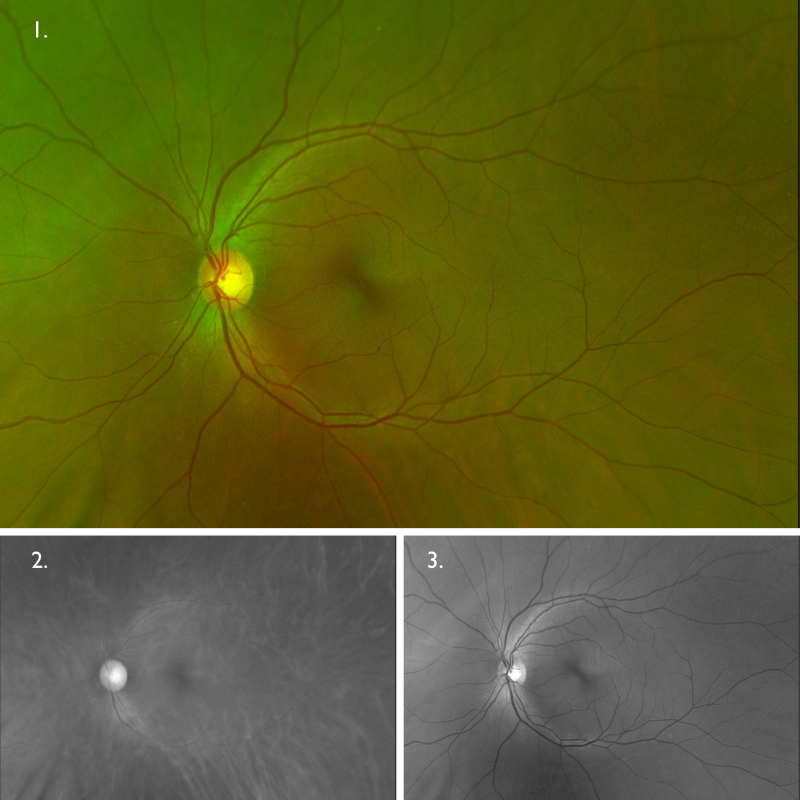
Optomap (1), red separation (2) and green separation (3) images – left eye: Widefield imaging of the left eye shows no apparent anomalies.
Fundus autofluorescence imaging – FAF (right and left eye): FAF shows an extensive area of hypo-autofluorescence colocalising with the area of retinal atrophy seen on clinical examination. The autofluorescence pattern for the left eye is unremarkable.
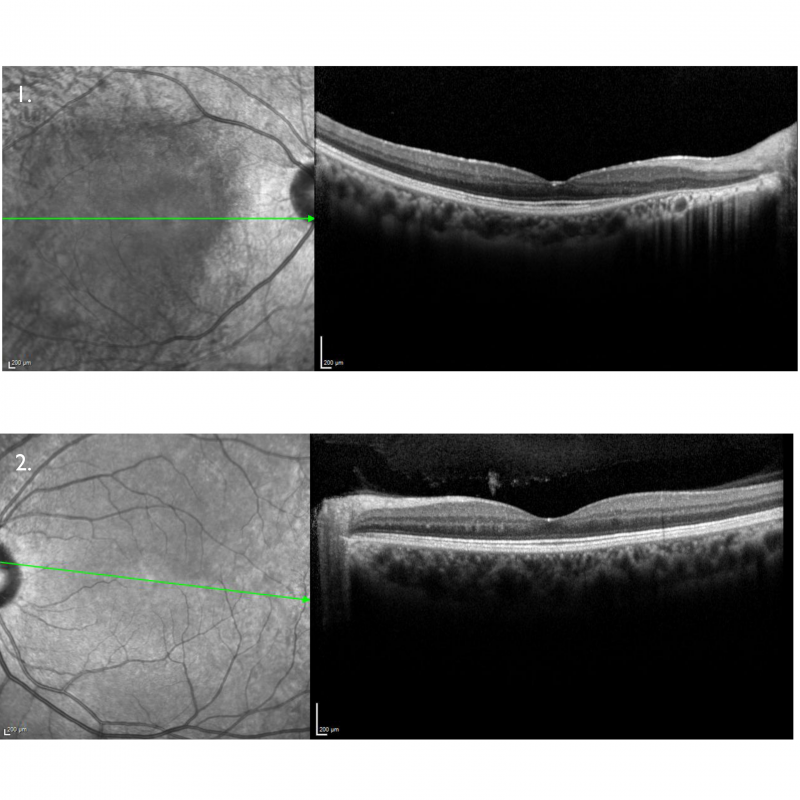
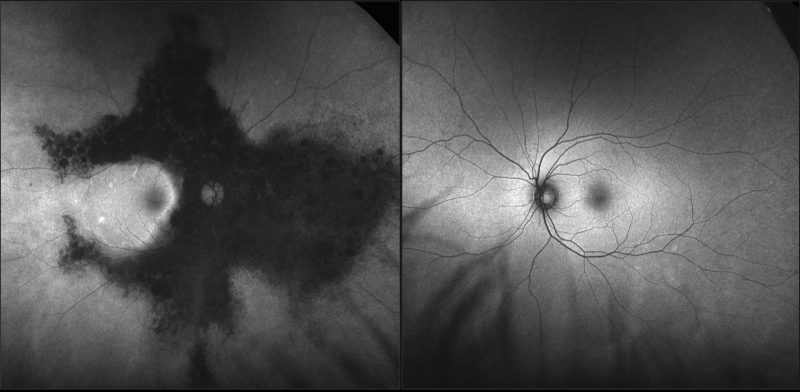
Spectralis OCT line scans – right (1) and left (2) macula: The right macular line scan shows atrophy of the outer retinal layers and RPE nasal to the foveal pit. The left macular line scan is unremarkable.
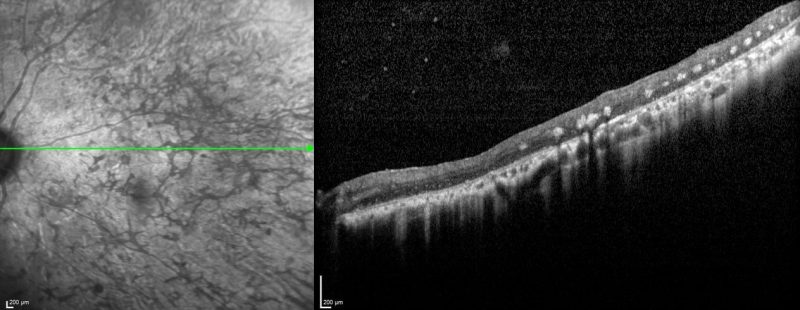
Spectralis OCT line scan – nasal retina (right eye): OCT scans through the areas of bone spicule pigmentation show atrophy of the outer retinal layers and hyper-reflective plaques corresponding to the pigmentation.
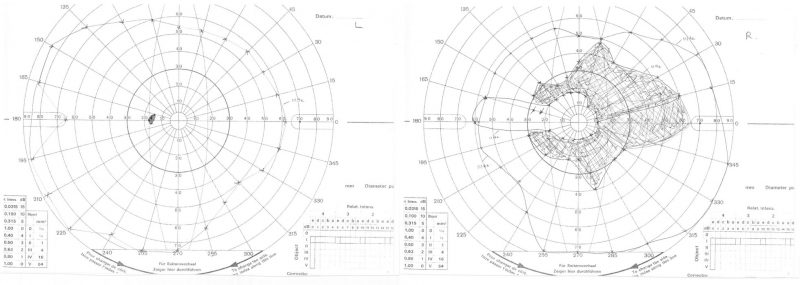
Goldmann kinetic perimetry: Shows a scotoma in the right visual field that corresponds to the area of retinal atrophy seen on widefield imaging. The left field is intact.
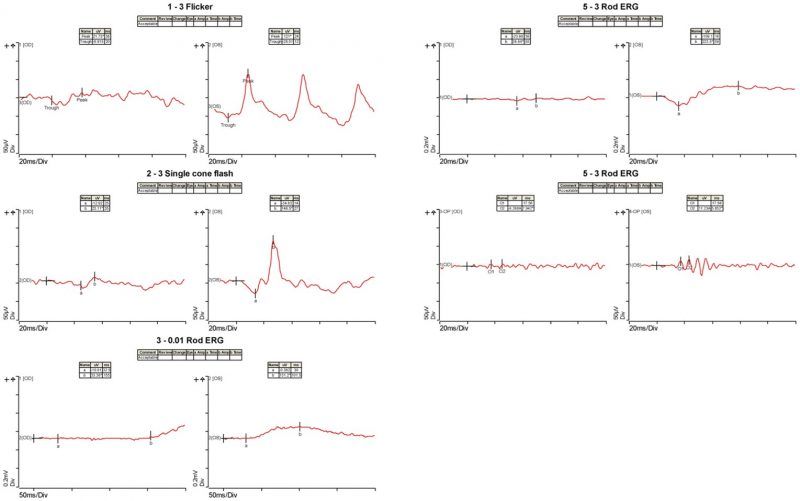
Electrophysiology results: Full-field ERG shows attenuated responses in the right eye and normal left eye response during both photopic and scotopic stimulations.
A 67-year-old Asian male with best-corrected visual acuity of 6/6 (20/20) in each eye. He has difficulty with night vision.
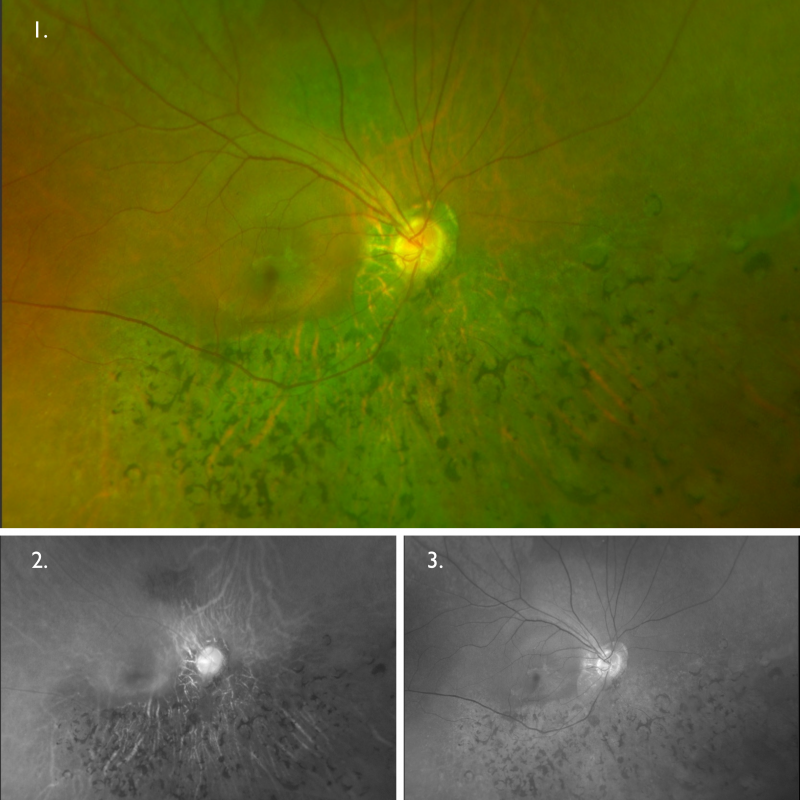
Optomap (1), red separation (2) and green separation (3) images – right eye: Widefield imaging shows bone spicule pigmentary changes in the inferior retina of the right eye. There is an isolated area of retinal mottling superior to the optic disc, otherwise, the superior retina is relatively unaffected.
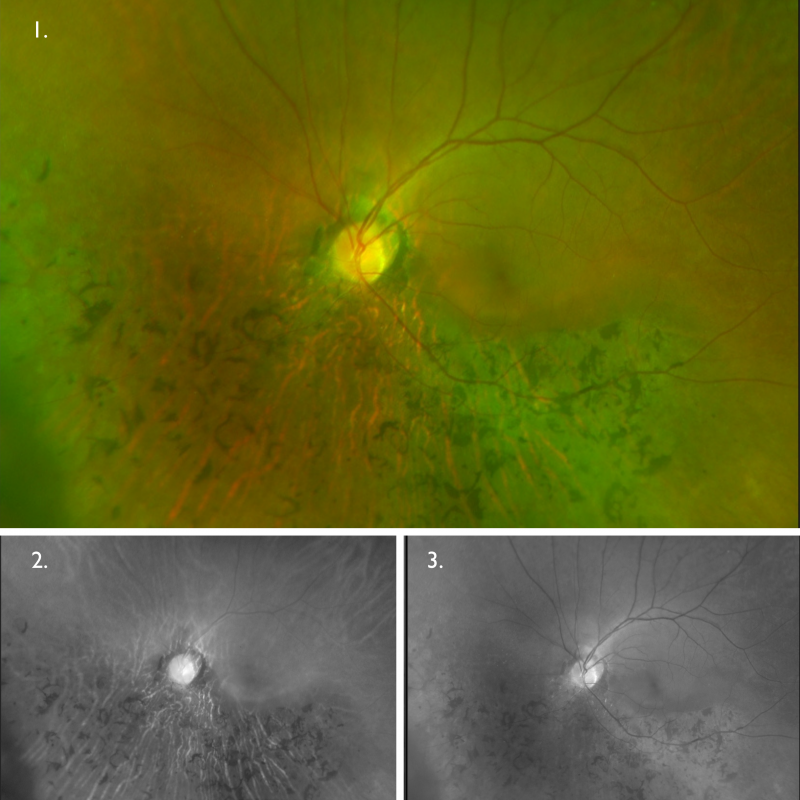
Optomap (1), red separation (2) and green separation (3) images – left eye
Widefield imaging shows bone spicule pigmentary changes in the inferior retina of the left eye. The superior retina appears relatively unaffected.
Differential Diagnosis
Gyrate Atrophy
Congenital Stationary Night Blindness (CSNB)
Bietti Crystalline Corneoretinal Dystrophy
Other differentials include pigmented paravenous chorioretinal atrophy, old retinal detachment, retinitis punctata albescens and acute zonal occult outer retinopathy (AZOOR). More information about these conditions can be found in the CFEH Atlas. Other conditions to consider may include drug-induced toxicity, chorioretinal infections, sequelae of inflammatory disease and Vitamin A deficiency.
References
Falfoul, Y., Elleuch, I., El Matri, K., Ghali, H., et al. (2020). Multimodal Imaging in Retinitis Pigmentosa: Correlations among Microvascular Changes, Macular Function and Retinal Structure. Journal of current ophthalmology. 32. 170-177.
Phelan, JK., Bok, D. (2000) A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Molecular Vision 2000; 6:116-24.
Sanne K. Verbakel, Ramon A.C. van Huet, Camiel J.F. Boon, Anneke I. den Hollander, Rob W.J. Collin, Caroline C.W. Klaver, Carel B. Hoyng, Ronald Roepman, B. Jeroen Klevering, (2018) Non-syndromic retinitis pigmentosa, Progress in Retinal and Eye Research, Volume 66, Pages 157-186.
Schuerch, K., Marsiglia, M., Lee, W., Tsang, S. H., & Sparrow, J. R. (2016). Multimodal imaging of disease associated pigmentary changes in retinitis pigmentosa. Retina 36 Suppl 1(Suppl 1), S147–S158.